Stereospecific anti SE2′ fluorination of allenylsilanes: synthesis of enantioenriched propargylic fluorides†
Abstract
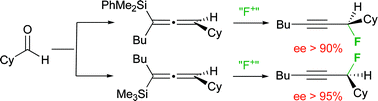
The electrophilic fluorodesilylation of enantioenriched allenylsilanes proceeds with efficient transfer of chirality. The silylation–

 Please wait while we load your content...
Please wait while we load your content...