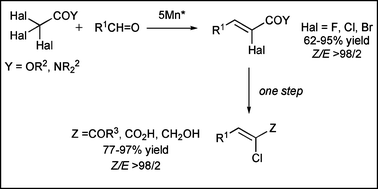
A Mn*-promoted sequential process directed toward the synthesis of (Z)-α-halo-α,β-unsaturated esters or amides is described. In both cases, the process takes place with complete Z-stereoselectivity. In addition, (Z)-α-chloro-α,β-unsaturated ketones and carboxylic acids, and (Z)-haloallylic alcohols were readily prepared from (Z)-α-halo-α,β-unsaturated amides derived from morpholine, or esters. A mechanism has been proposed to explain the sequential process and the stereoselectivity observed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...