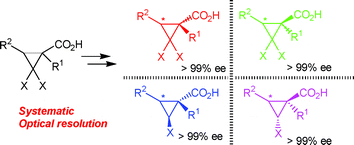
We performed an efficient practical and systematic optical resolution method for gem-dihalo- and monohalocyclopropanecarboxylic acids 1 and 5 utilizing chiral 1,1′-binaphthol monomethyl ether (R)-2 as the key auxiliary. Direct esterification of 1 with (R)-2 gave two 1R- and 1S-diastereomeric esters3 with marked different Rfvalues, both of which were easily separated using simple column chromatography. Monodehalogenation of separated chiral esters3 using t-BuMgCl and cat. Co(dppe)2Cl2 gave two 1,2-trans- and 1,2-cis-diastereomers4 with markedly different Rf values, both of which were similarly separated using simple column chromatography. The obtained diastereomers 3 and 4 were easily hydrolyzed to the desired enantiopure acids 1 (>99%) and 5 (>99%), respectively, with recovery of (R)-2, both in good to excellent yields. Utilizing the present method, important chiral agrochemicals, carpropamid6 and fencyclate7, were readily synthesized. Pyrethroid 9 with three asymmetric centers was efficiently synthesized in a much better yield compared with the reported method.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...