Taking control of P1, P1′ and double bond stereochemistry in the synthesis of Phe-Phe (E)-alkene amide isostere dipeptidomimetics†
Abstract
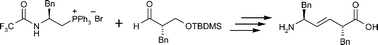
A protocol for the stereocontrolled independent preparation of both C-2 epimers of Phe-Phe trans-vinyl amide isostere dipeptidomimetics has been devised based on a Wittig-type reaction, in which two chiral building blocks were joined with excellent E-selectivity to give compounds of the type PheΨ[(E)-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH]-PheOH.
CH]-PheOH.

 Please wait while we load your content...
Please wait while we load your content...