Synthesis and properties of nicked dumbbell and dumbbell DNA conjugates having stilbenedicarboxamide linkers
Abstract
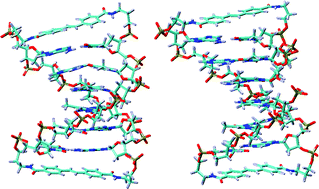
The synthesis and properties of nicked dumbbell and dumbbell DNA conjugates having A-tract base pair domains connected by rod-like stilbenedicarboxamide linkers are reported. The nicked dumbbells have one to eight dA–dT base pairs and are missing a sugar–

 Please wait while we load your content...
Please wait while we load your content...