Substrate specificity in enzymatic fluorination. The fluorinase from Streptomyces cattleya accepts 2′-deoxyadenosine substrates†
Abstract
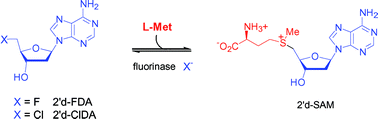
The fluorinase enzyme from Streptomyces cattleya displays an unusual ability in biocatalysis in that it forms a C–F bond. We now report that the enzyme will accept

 Please wait while we load your content...
Please wait while we load your content...