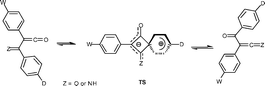
1,3-Phenyl shifts interconvert imidoylketenes 1 and α-oxoketenimines 2 and, likewise, α-oxoketenes 3 automerize by this 1,3-shift. These rearrangements usually take place in the gas phase under conditions of flash vacuum thermolysis. Energy profiles calculated at the B3LYP/6-31G(d,p) and B3LYP/6311 + G(3df,2p)//B3LYP/6-31G(d,p) levels demonstrate that electron donating substituents (D) in the migrating phenyl group and electron withdrawing ones (W) in the non-migrating phenyl group, can stabilise the transition states TS1 and TS2 to the extent that activation barriers of ca. 100 kJ mol−1 or less are obtained; i.e. enough to make these reactions potentially observable in solution at ordinary temperatures. The calculated transition state energies ΔG(TS1) show an excellent correlation with the Hammett constants σp(W) and σp+(D).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...