(S,S)-(+)-Pseudoephedrine as chiral auxiliary in asymmetric acetate aldol reactions
Abstract
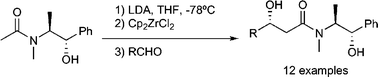
The asymmetric acetate-type aldol reaction using (S,S)-(+)-pseudoephedrine has been studied in detail. Experimental variables like the nature of the metal counterion of the enolate, the presence of additives and the structure of the

 Please wait while we load your content...
Please wait while we load your content...