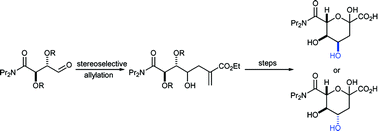
The stereoselective synthesis of two epimeric screening substrates, (4R, 5R, 6R)- and (4S, 5R, 6R)-6-dipropylcarbamoyl-2-oxo-4,5,6-trihydroxy-hexanoic acid, for the directed evolution of sialic acid aldolase is described. The complementary methods relied on stereoselective indium-mediated additions of ethyl α-bromomethyl acrylate to functionalised aldehydes. With an α-hydroxy aldehyde, (2R, 3R)-2,3-dihydroxy-4-oxo butanoic acid dipropylamide, the addition was chelation controlled, and the syn product, (6R, 5R, 4S)-6-dipropylcarbamoyl-2-methylidene-4,5,6-trihydroxy-hexanoic acid ethyl ester, was obtained. In contrast, the stereochemical outcome of the addition to (2R, 3R)-N,N-dipropyl-2,3-O-isopropylidene-4-oxobutyramide was consistent with Felkin–Anh control, and the anti adduct, (4R, 5R, 6R)-6-dipropylcarbamoyl-2-methylidene-4-hydroxy-5,6-O-isopropylidene-hexanoic acid ethyl ester, was the major product. Ozonolysis and deprotection gave the screening substrates as mixtures of furanose and pyranose forms, in good yields.

 Please wait while we load your content...
Please wait while we load your content...