Novel monoazacryptand-type fluorescent chemosensors, 1
(derived from an 18-crown-6) and 2
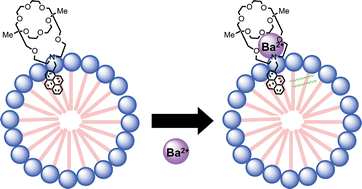
(derived from a 15-crown-5) both with a pyrene ring as their photoresponsive moiety, were synthesized. Their fluorescence properties for alkali metal and alkaline earth metal cations in water were then examined. The detection of metal cations was accomplished by a change in the fluorescence intensity of the host compounds, based on a photoinduced electron transfer (PET) mechanism. In aqueous solution, 1 showed little fluorescence upon the addition of Ba2+ because of the very weak complexation with Ba2+, but the presence of micelles of polyoxyethylene(10) isooctylphenyl ether (Triton X-100) enabled 1 to show highly sensitive and selective Ba2+ detection among alkali metal and alkaline earth metal cations. With respect to the selective fluorescent detection of important metal cations (Na+, K+, Mg2+, Ca2+) relevant to living organisms, 2 was found to detect K+ with high selectivity in aqueous micellar solutions of polyoxyethylene(20) sorbitan monostearate (Tween-60). The selectivity for metal cations was mainly dependent on the goodness of fit of the host cavity and the metal cation size. In the presence of anionic surfactants, 2 detected alkaline earth metal cations more effectively than alkali metal cations.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...