7-N,7′-N′-(1″,2″-Dithianyl-3″,6″-dimethylenyl)bismitomycin C: synthesis and nucleophilic activation of a dimeric mitomycin†
Abstract
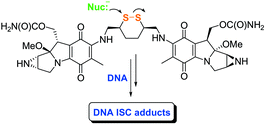
Dimeric alkylating agents that modify complementary DNA strands have engendered significant interest. We have prepared the novel dimeric mitomycin, 7-N,7′-N′-(1″,2″-dithianyl-3″,6″-dimethylenyl)bismitomycin C (9), in which the mitomycins are bridged by a dithiane unit. Dimer 9, like the clinically tested acyclic disulfides KW-2149 (3) and BMS-181174 (4), was designed to activate under nucleophilic and reductive conditions. Successive nucleophile-mediated disulfide cleavage transformations of 9 are expected to generate thiol species ideally positioned to render the two mitomycin systems vulnerable to nucleophilic attack and permit DNA interstrand cross-link formation. The dithiane linker, strategically positioned between the two mitomycins, distinguished 9 from 3 and 4. Nucleophilic activation of this cyclic disulfide permitted both activated mitomycins to remain tethered to one another. We report the synthesis of 9, and show that the nucleophile Et3P markedly enhances the activation and consumption of 9, compared with the reference compound 7-N, 7′-N′-(cyclohexanyl-trans-1″,4″-dimethylenyl)bismitomycin C (27). We further demonstrated that 9 provides higher levels of DNA interstrand cross-links than either the dimeric reference compounds, 27 and 7-N,7-N′-(2″,5″-dihydroxy-1″,6″-hexanediyl)bismitomycin C (28), or the monomeric mitomycins, 1 and 3, when Et3P is added to solutions containing EcoRI-linearized pBR322 DNA.

 Please wait while we load your content...
Please wait while we load your content...