The chirality of dendrimer-based supramolecular complexes
Abstract
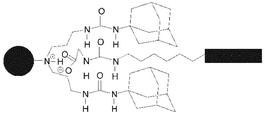
Boc-protected L-phenylalanine has been connected to a spacer-armed ureido–acetic acid derivative, which can form multiple supramolecular complexes with urea–adamantyl modified poly(propylene imine) dendrimers in

 Please wait while we load your content...
Please wait while we load your content...