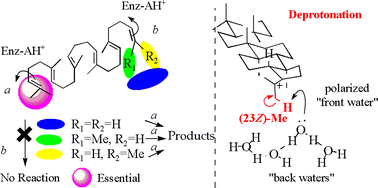
To provide insight into the polycyclization mechanism of squalene by squalene–hopene cyclase (SHC) from Alicyclobacilus acidocaldarius, some analogs of nor- and bisnorsqualenes were synthesized including the deuterium-labeled squalenes and incubated with the wild-type SHC, leading to the following inferences. (1) The deprotonation reaction for the introduction of the double bond of the hopene skeleton occurs exclusively from the Z-methyl group on the terminal double bond of squalene. (2) 3R-Oxidosqualene was folded in a boat conformation for the A-ring construction, while the 3S-form was in a chair structure. (3) The terminal two methyl groups are indispensable both for the formation of the 5-membered E-ring of the hopene skeleton and for the initiation of the polycyclization cascade, but the terminal Z-methyl group has a more crucial role for the construction of the 5-membered E-ring than the E-methyl group. (4) Some of the novel terpene skeletons, 36, 37, 39 and 40, were created from the analogs employed in this investigation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...