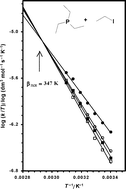
Accurate second-order rate constants were measured at 5 K intervals in the temperature range 298.15–328.15 K for the quaternisation reaction of triethylphosphine with iodoethane in methanol, ethanol, propan-1-ol and butan-1-ol. These data are complemented previously reported rate constants for the quaternisation reaction of triethylamine with iodoethane in the same solvents and at similar temperatures. Each of these two reaction series is analysed in terms of the isokinetic relationship (IKR) with respect to solvent variation and of the isosolvent relationship (ISoR) with respect to temperature variation, using in the latter case five different empirical solvent scales. Statistically validated IKR and ISoR have been found for both reaction series. The resulting isokinetic temperatures of 347 K (phosphine series) and of 730 K (amine series) are discussed in terms of Linert's theory of the isokinetic relationship. The best ISoR correlation is obtained using the Dimroth–Reichardt ETN solvent scale for the phosphine series and the Kamlet–Taft αKT solvent scale for the amine series. It is demonstrated that no real solvent can be envisaged as having the characteristics of an isokinetic solvent. The selectivity of the nucleophiles triethylphosphine and triethylamine in the attack on iodoethane is examined by treating together both reaction series in terms of the isoselective relationship (ISeR). The isoselective temperature with respect to solvent is found to be 289 K, which is close to the value of 302 K predicted by Exner and Giese's formula on the basis of the individual isokinetic temperatures. A novel ISeR analysis with respect to temperature is performed. It reveals that the αKT scale is the most appropriate solvent scale for describing this selectivity series, and that it is feasible to find an isoselective solvent. A new equation is developed for predicting the isoselective solvent parameter from individual isosolvent parameters and is shown to yield realistic values. The present similarity analysis shows that there are significant differences between the courses of these quaternisation reactions. On the basis of the experimentally determined isoparameter values, in liquid alcohols as solvent it is proposed that the reaction between triethylphosphine and iodoethane follows a classic bimolecular nucleophilic substitution pathway, whereas the desolvation of triethylamine molecules has to be taken into account to describe the mechanism of the original Menshutkin reaction.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...