A total synthesis of (±)-phomactin A
Abstract
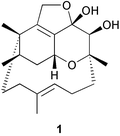
A total synthesis of the PAF antagonist phomactin A (1), isolated from the marine fungus Phoma sp. is described. The synthesis is based on a Cr(II)/Ni(II) macrocyclisation from the aldehyde vinyl iodide 14, leading to the key phomactatrienol intermediate 16a, followed by elaboration of 16a to the epoxyketone 21, which undergoes spontaneous pyran and hemiacetal ring formation to 1 on deprotection with DDQ.

 Please wait while we load your content...
Please wait while we load your content...