Synthesis and evaluation of new potential HIV-1 non-nucleoside reverse transcriptase inhibitors. New analogues of MKC-442 containing Michael acceptors in the C-6 position
Abstract
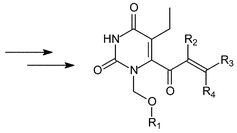
Analogues of MKC-442 capable of undergoing Michael addition reactions were synthesised in order to investigate the activity against the HIV-1 mutant (Y181C). An improved activity was postulated on the basis of a possible covalent binding to the mercapto group of Cys181. Lithiation of the C-6 position of 1-ethoxymethyl-5-ethyl-1H-pyrimidine-2,4-dione (5) was followed by reaction with α,β-unsaturated

 Please wait while we load your content...
Please wait while we load your content...