Synthetic studies towards the tunicamycins and analogues based on diazo chemistry. Total synthesis of tunicaminyl uracil
Abstract
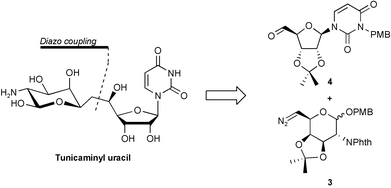
A synthetic approach to the tunicamycins, a complex family of nucleosides with potent antibiotic and antiviral activities is reported based on diazo chemistry. The corresponding precursors for the synthesis of tunicaminyl uracil derivatives, the non-stabilized diazo derived from 13 and the aldehyde derivative of uridine, compound 4, were prepared efficiently from commercially available D-galactal and uridine, respectively. After a high yielding coupling reaction to obtain the ketone 14, a stereoselective reduction provided the corresponding tunicaminyl uracil derivative 17a and its C-7 epimer 17b. The interconversion of the diazo and aldehyde functional groups in the requisite building blocks was similarly achieved to obtain the ketone 32, which after reduction yielded the corresponding 7-deoxy-6-hydroxy tunicaminyl uracil analogs 33a and 33b.

 Please wait while we load your content...
Please wait while we load your content...