4-Fluorinated l-lysine analogs as selective i-NOS inhibitors: methodology for introducing fluorine into the lysine side chain†‡
Abstract
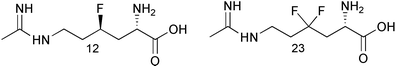
In the literature, the introduction of fluorine into bioactive molecules has been known to enhance the biological activity relative to the parent molecule. Described in this article is the synthesis of 4R-fluoro-L-NIL (12) and 4,4-difluoro-L-NIL (23) as part of our iNOS program. Both 12 and 23 were found to be selective iNOS inhibitors as shown in

 Please wait while we load your content...
Please wait while we load your content...