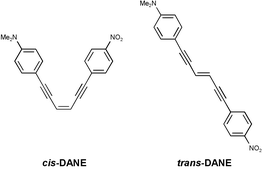
trans- and cis-1-(4-Dimethylaminophenyl)-6-(4-nitrophenyl)hex-3-ene-1,5-diynes (trans- and cis-DANE) were synthesized and their photochemical properties were studied. The absorption spectra of trans-DANE red-shifted compared with the parent compound bisphenylethynylethene (BEE) due to intramolecular charge transfer. The fluorescence spectra, Stokes shift, fluorescence lifetime, fluorescence quantum yield, and quantum yield of trans-to-cis photoisomerization of trans-DANE showed strong dependence upon the solvent polarity in the less-polar region. No fluorescence emission from trans-DANE was observed in medium-polar and polar solvents. The quantum yield of cis-to-trans isomerization was almost solvent independent. The donor–acceptor substituents shifted the equilibrium between the trans perpendicular triplet state and the trans planar triplet state to the trans triplet state, and resulted in an increase in the triplet lifetime. Comparison of the photochemical properties of trans-DANE with trans-4-dimethylamino-4′-nitrostilbene (DANS) suggests that trans-DANE is a possible fluorescent probe in the non-polar region.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...