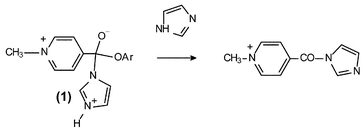
Reaction of imidazole with toluene-4-sulfonate salts of substituted phenyl N-methylpyridinium-4-carboxylate esters: special base catalysis by imidazole
Abstract
The reaction of imidazole in aqueous solution with toluene-4-sulfonate salts of substituted phenyl N-methylpyridinium-4-carboxylate esters obeys the rate law:

 Please wait while we load your content...
Please wait while we load your content...