The development and preparation of the 2,4-dimethoxybenzyl arylhydrazine (DMBAH) “latent” safety-catch linker: solid phase synthesis of ketopiperazines
Abstract
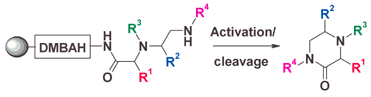
The development and preparation of the 2,4-dimethoxybenzyl arylhydrazine (DMBAH) linker 3, a new class of “latent” safety-catch linker which is stable under Mitsunobu alkylation conditions and in the presence of amines and hydrazine, is reported. The utility of the new linker is exemplified by the synthesis of ketopiperazines (MKPs) 24 bearing up to four points of diversity using a cyclitive cleavage approach.

 Please wait while we load your content...
Please wait while we load your content...