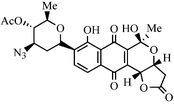
The synthesis of an isomeric mixture of 4-O-acetyl-3-azido-2,3,6-trideoxy-β-D-arabino-hexopyranosyl analogues 6 of the C-glycosylpyranonaphthoquinone antibiotic medermycin is described. The key 3-acetyl-6-(4-O-acetyl-3-azido-2,3,6-trideoxy-β-D-arabino-hexopyranosyl)-5-methoxy-1,4-naphthoquinone 8 was prepared via Stille coupling of 6-(3-azido-2,3,6-trideoxy-β-D-arabino-hexopyranosyl)-3-bromo-1,4-naphthoquinone 17 with (α-ethoxyvinyl)tributylstannane followed by hydrolysis and oxidation of the resultant hydroquinone 18. Bromonaphthoquinone 17 in turn was afforded by oxidative demethylation of 6-(4-O-acetyl-3-azido-2,3,6-trideoxy-β-D-arabino-hexopyranosyl)-3-bromo-1,4,5-trimethoxynaphthalene 16 formed by regioselective bromination of 6-(4-acetyl-3-azido-2,3,6-trideoxy-β-D-arabino-hexopyranosyl)-1,4,5-trimethoxynaphthalene 10. This latter naphthalene 10 was prepared via direct C-glycosylation of naphthol 12 with glycosyl donor 11 using BF3·Et2O in acetonitrile. The regioselectivity of the bromination of naphthalene 10 was independently determined by reductive monomethylation of the 6-(4-O-acetyl-3-azido-2,3,6-trideoxy-β-D-arabino-hexopyranosyl)-5-methoxy-1,4-naphthoquinone 22 to naphthol 23 followed by selective ortho bromination to bromide 24 and methylation to 16. Attempts to effect acetylation of 6-(4-O-acetyl-3-azido-2,3,6-trideoxy-β-D-arabino-hexopyranosyl)-3-bromo-1,4,5-trimethoxynaphthalene 16 and 3-bromo-6-(3-dimethylamino-2,3,6-trideoxy-β-D-arabino-hexopyranosyl)-1,4,5-trimethoxynaphthalene 26via Stille coupling with (α-ethoxyvinyl)tributylstannane were low yielding thereby establishing the necessity to use an azido group as a latent dimethylamino group and a more electrophilic bromonaphthoquinone as the coupling partner for the Stille reaction. Addition of 2-trimethylsilyloxyfuran 9 to 3-acetyl-6-(4-O-acetyl-3-azido-2,3,6-trideoxy-β-D-arabino-hexopyranosyl)-5-methoxy-1,4-naphthoquinone 8 afforded the furofuran adducts 7 and 19 as an inseparable mixture of diastereomers. Oxidative rearrangement of this diastereomeric mixture using ceric ammonium nitrate afforded the inseparable diastereomeric furonaphthopyrans 6 and 20.

 Please wait while we load your content...
Please wait while we load your content...