DOTTADs – readily made novel metal ligands with multivariant functionality
Abstract
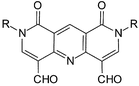
The interaction of Hantzsch pyridinecarboxylic acids with dialkylformamides and POCl3, followed by treatment with NH4OH yields 1,8-dioxo-1,2,7,8-tetrahydro-2,7,10-triazaanthracenes (DOTTADs), which have great potential as useful ligands for Group I and II metals and some transition metals. The corresponding Hantszch esters similarly give DOTTADs or their bis-imines by way of isolable intermediates, which are then treated with an amine RNH2. DOTTAD-imines are also available from DOTTADs with amines and this reaction is also effective with 1,8-diamino-3,6-dioxaoctane to give macrocyclic analogues, as well as with chiral amines. DOTTAD-imines can be reduced with diethylsilane and Wilkinson's catalyst to the corresponding amines, which can also be formed from DOTTADs by reductive amination with amines and Na(AcO)3BH. Mono-aldols of DOTTADs are easily formed by treatment of DOTTADs with acetone.

 Please wait while we load your content...
Please wait while we load your content...