Deoxygenation of carbohydrates by thiol-catalysed radical-chain redox rearrangement of the derived benzylidene acetals
Abstract
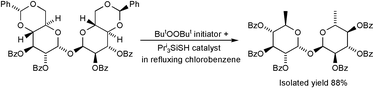
Five- or six-membered cyclic benzylidene acetals, derived from 1,2- or 1,3-diol functionality in carbohydrates, undergo an efficient thiol-catalysed radical-chain redox rearrangement resulting in deoxygenation at one of the diol termini and formation of a benzoate ester function at the other. The role of the thiol is to act as a protic polarity-reversal catalyst to promote the overall abstraction of the acetal hydrogen atom by a nucleophilic alkyl radical. The redox rearrangement is carried out in refluxing octane and/or chlorobenzene as solvent at ca. 130 °C and is initiated by thermal decomposition of di-tert-butyl peroxide (DTBP) or 2,2-bis(tert-butylperoxy)butane. The silanethiols (ButO)3SiSH and Pri3SiSH (TIPST) are particularly efficient catalysts and the use of DTBP in conjunction with TIPST is generally the most effective and convenient combination. The reaction has been applied to the monodeoxygenation of a variety of monosaccharides by way of 1,2-, 3,4- and 4,6-O-benzylidene pyranoses and a 5,6-O-benzylidene furanose. It has also been applied to bring about the dideoxygenation of mannose and of the disaccharide α,α-trehalose. The use of p-methoxybenzylidene acetals offers no great advantage and ethylene acetals do not undergo significant redox rearrangement under similar conditions. Functional group compatibility is good and tosylate, epoxide and ketone functions do not interfere; it is not necessary to protect free OH groups. Because of the different mechanisms of the ring-opening step (homolytic versus heterolytic), the regioselectivity of the redox rearrangement can differ usefully from that resulting from the Hanessian–Hullar (H.–H.) and Collins reactions for brominative ring opening of benzylidene acetals. When simple deoxygenation of a carbohydrate is desired, the one-pot redox rearrangement offers an advantage over H.–H./Collins-based procedures in that the reductive debromination step (which often involves the use of toxic tin hydrides) required by the latter methodology is avoided.

 Please wait while we load your content...
Please wait while we load your content...