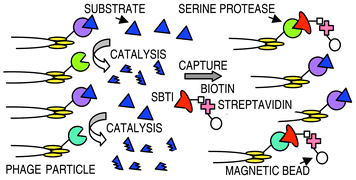
A library of blood coagulation factor Xa
(FXa)–trypsin hybrid proteases was generated and displayed on phage for selection of derivatives with the domain “architecture” of trypsin and the specificity of FXa. Selection based on binding to soybean trypsin inhibitor only provided enzymatically inactive derivatives, due to a specific mutation of serine 195 of the catalytic triad to a glycine, revealing a significant selection pressure for proteolytic inactive derivatives. By including a FXa peptide substrate in the selection mixture, the majority of the clones had retained serine at position 195 and were enzymatically active after selection. Further, with the inclusion of bovine pancreatic trypsin inhibitor, in addition to the peptide substrate, the selected clones also retained FXa specificity after selection. This demonstrates that affinity selection combined with appropriate deselection provides a simple strategy for selection of enzyme derivatives that catalyse a specific reaction.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...