Synthesis, biological activity, and conformational analysis of CD-ring modified trans-decalin 1α,25-dihydroxyvitamin D analogs†
Abstract
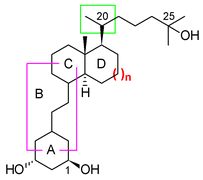
A novel series of analogs of 1,25-dihydroxyvitamin D3, the hormonally active metabolite of vitamin D3, characterised by the presence of a trans-fused decalin CD-ring system, possesses surprising biological activities in combination with specific structural modifications in the flexible parts of the molecule, when compared with the natural hydrindane derivatives. (1) A large difference in biological activity is observed between the 20-epimeric trans-decalin analogs that follows a pattern opposite to what is usually observed for the natural ring size. (2) Several trans-decalin analogs that are modified in the seco-B-ring region, including previtamin derivatives, possess a pronounced vitamin D-like activity, whereas the corresponding hydrindane derivatives are inactive. The molecular origin of this behavior is still under study.

 Please wait while we load your content...
Please wait while we load your content...