Isofagomine lactams, synthesis and enzyme inhibition
Abstract
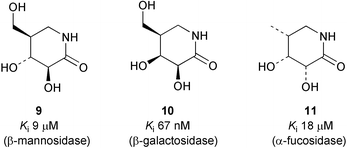
The synthesis of isofagomine lactams (2-oxoisofagomines) corresponding to the biologically important hexoses is presented. The D-glucose/D-mannose analogue (3S,4R,5R)-3,4-dihydroxy-5-hydroxymethylpiperidin-2-one (9) was synthesised in 9 steps from D-arabinose, the D-galactose analogue (3S,4S,5R)-3,4-dihydroxy-5-hydroxymethylpiperidin-2-one (10) was synthesised in 11 steps from D-arabinose and the L-fucose analogue (3R,4R,5R)-3,4-dihydroxy-5-methylpiperidin-2-one (11) was synthesised in 12 steps from L-arabinose. The three lactams 9–11 were found to be glycosidase inhibitors with micro- to nanomolar inhibition constants. The lactam 10 showed slow onset inhibition of β-galactosidase from A. Oryzae. The rate constants for this process were determined to be kon = 2.55 × 104 M−1 s−1 and koff = 1.7 × 10−3 s−1. The activation energies and standard thermodynamic functions were also determined.

 Please wait while we load your content...
Please wait while we load your content...