A convergent approach to the marine natural product eleutherobin: synthesis of key intermediates and attempts to produce the basic skeleton
Abstract
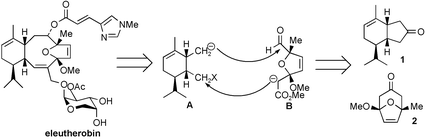
Synthesis of (1R,5R,6R)-2-(6-hydroxymethyl-5-isopropyl-2-methylcyclohex-2-enyl)-N-methoxy-N-methylacetamide 8 from R-(−)-phellandrene in six steps, and (3aR*,4S*,6R*,6aS*)-(6-hydroxymethyl-4-methoxy-2,2,6-trimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)acetic acid methyl ester 17 from tetrabromoacetone and 2-methoxy-5-methylfuran in six steps, provided two key fragments which have been combined to produce intermediates for attempted construction of the basic skeleton of eleutherobin.

 Please wait while we load your content...
Please wait while we load your content...