Boosting water oxidation electrocatalysts with surface engineered amorphous cobalt hydroxide nanoflakes†
Abstract
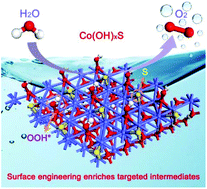
Enriching dominant active intermediates is most pivotal in developing efficient non-noble oxygen evolution reaction (OER) electrocatalysts for water oxidation. Herein, we report surface-engineered amorphous cobalt hydroxide nanoflakes on nickel foam as highly active electrocatalysts for boosting water oxidation by a new repeatedly switching current–polarity strategy. It is discovered that sulfur introduction can simultaneously increase the Co3+/Co2+ ratio to generate more targeted OOH* intermediates and regulate the surface electronic structure to greatly boost its intrinsic activity. The density functional theory (DFT) calculations further confirm the reduction of the free energy of the OOH* intermediates. Consequently, our Co(OH)xS electrocatalyst exhibits an ultralow overpotential of 283 and 365 mV at 100 and 1000 mA cm−2 in alkaline media, respectively, and its turnover frequency (TOF) is more than 4 times higher than the corresponding Co(OH)x catalysts. This heteroatom triggered surface engineering may open up avenues to explore other efficient non-noble metal electrocatalysts for water oxidation.


 Please wait while we load your content...
Please wait while we load your content...