Oxygen incorporated WS2 nanoclusters with superior electrocatalytic properties for hydrogen evolution reaction†
Abstract
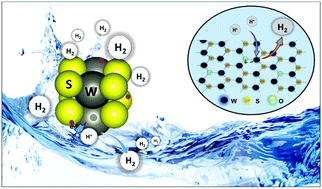
Transition metal dichalcogenides (TMDs) exhibit unique properties and show potential for promising applications in energy conversion. Mono/few-layered TMDs have been widely explored as active electrocatalysts for the hydrogen evolution reaction (HER). A controlled synthesis of TMD nanostructures with unique structural and electronic properties, leading to highly active sites or higher conductivity, is essential to achieve enhanced HER activity. Here, we demonstrate a new approach to controllably synthesize highly catalytically active oxygen-incorporated 1T and 2H WS2 nanoclusters from oxygen deficient WO3 nanorods, following chemical exfoliation and ultrasonication processes, respectively. The as-synthesized 1T nanoclusters, with unique properties of tailored edge sites, and enhanced conductivity resulting from the metallic 1T phase and oxygen incorporation, have been identified as highly active and promising electrocatalysts for the HER, with a very low Tafel slope of 47 mV per decade and a low onset overpotential of 88 mV, along with exceptionally high exchange current density and very good stability. The study could be extended to other TMD materials for potential applications in energy conversion and storage.


 Please wait while we load your content...
Please wait while we load your content...
