Borophene hydride: a stiff 2D material with high thermal conductivity and attractive optical and electronic properties
Abstract
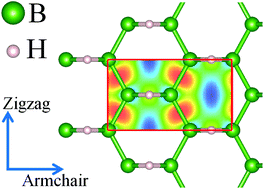
Two-dimensional (2D) structures of boron atoms, so-called borophene, have recently attracted remarkable attention. In a recent exciting experimental study, a hydrogenated borophene structure was realized. Motivated by this success, we conducted extensive first-principles calculations to explore the mechanical, thermal conduction, electronic and optical responses of borophene hydride. The mechanical response of borophene hydride was found to be anisotropic, with an elastic modulus of 131 N m−1 and a high tensile strength of 19.9 N m−1 along the armchair direction. Notably, it was shown that by applying mechanical loading the metallic electronic character of borophene hydride can be altered to direct band-gap semiconducting, very appealing for application in nanoelectronics. The absorption edge of the imaginary part of the dielectric function was found to occur in the visible range of light for parallel polarization. Finally, it was estimated that this novel 2D structure at room temperature can exhibit high thermal conductivities of 335 W mK−1 and 293 W mK−1 along the zigzag and armchair directions, respectively. Our study confirms that borophene hydride shows an outstanding combination of interesting mechanical, electronic, optical and thermal conduction properties, which are promising for the design of novel nanodevices.


 Please wait while we load your content...
Please wait while we load your content...