A chelation-induced cooperative self-assembly methodology for the synthesis of mesoporous metal hydroxide and oxide nanospheres†
Abstract
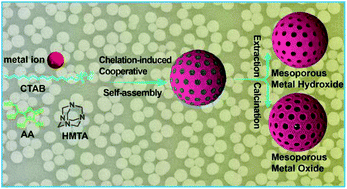
With unique physical and chemical properties and porous architectures, mesoporous transition metal hydroxide (MMHO) and oxide (MMO) nanospheres hold great potential for various applications in drug delivery, catalysis, energy storage and conversion. However, synthesizing MMHO and MMO with well-defined mesostructures remains a great challenge because of the weak interaction between surfactants and metal precursors. Herein we describe a chelation-induced cooperative self-assembly system in which the weak interaction can be cooperatively amplified through the use of a chelating ligand acting as a co-template. Both MMHO and MMO nanospheres with tunable diameters and high surface areas can be readily synthesized via this strategy. The as-synthesized mesoporous ZnO nanospheres exhibit excellent photoelectric performance, and as a highly efficient oxygen evolution reaction (OER) catalyst of low cost, the calcined Cu(OH)2 nanospheres exhibit one of the best activities for the OER. Moreover, this cooperative method gives rise to an alternative to “classical” self-assembly methods for the preparation of mesostructured nanomaterials and, in some cases, the only viable synthetic route toward MMHO and MMO nanostructures.


 Please wait while we load your content...
Please wait while we load your content...