Di-isopropyl ether assisted crystallization of organic–inorganic perovskites for efficient and reproducible perovskite solar cells†
Abstract
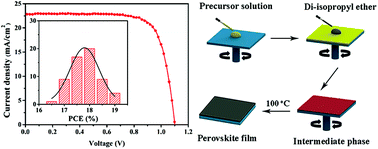
Organic–inorganic perovskite solar cells have emerged as a promising photovoltaic technology because of their advantages such as low cost, high efficiency, and solution processability. The performance of perovskite solar cells is highly dependent on the crystallinity and morphology of the perovskite films. Herein, we report a simple, one-step anti-solvent deposition process using di-isopropyl ether as a dripping solvent to obtain extremely uniform and highly crystalline CH3NH3PbI3 perovskite films. Compared to toluene, chlorobenzene, chloroform, or diethyl ether, di-isopropyl ether has proven to be a more suitable solvent for an anti-solvent deposition process. The perovskite solar cells fabricated by the anti-solvent deposition process using di-isopropyl ether treatment exhibit an average power conversion efficiency (PCE) of 17.67 ± 0.54% and the highest PCE of 19.07%. Moreover, the higher boiling point of di-isopropyl ether makes the anti-solvent deposition process more tolerant to elevated ambient temperature, which can be carried out at ambient temperatures up to 40 °C. Our results demonstrate that di-isopropyl ether is an excellent dripping solvent in the anti-solvent deposition process for efficient and reproducible perovskite solar cells.


 Please wait while we load your content...
Please wait while we load your content...