The impact of metalation on adsorption geometry, electronic level alignment and UV-stability of organic macrocycles on TiO2(110)†
Abstract
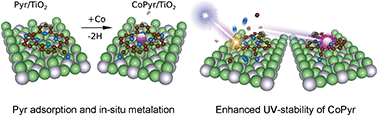
Metal complexes of the tetradentate bipyridine based macrocycle pyrphyrin (Pyr) have recently shown promise as water reduction catalysts in homogeneous photochemical water splitting reactions. In this study, the adsorption and metalation of pyrphyrin on stoichiometric TiO2(110) is investigated in ultrahigh vacuum by means of scanning tunneling microscopy, photoelectron spectroscopy, low-energy electron diffraction, and density functional theory. In a joint experimental and computational effort, the local adsorption geometry at low coverage, the long-range molecular ordering at higher coverage and the electronic structure have been determined for both the bare ligand and the cobalt-metalated Pyr molecule on TiO2. The energy level alignment of CoPyr/TiO2 supports electron injection into TiO2 upon photoexcitation of the CoPyr complex and thus renders it a potential sensitizer dye. Importantly, Co-incorporation is found to stabilize the Pyr molecule against photo-induced degradation, while the bare ligand is decomposed rapidly under continuous UV-irradiation. This interesting phenomenon is discussed in terms of additional de-excitation channels for electronically highly excited molecular states.


 Please wait while we load your content...
Please wait while we load your content...