Planar B38− and B37− clusters with a double-hexagonal vacancy: molecular motifs for borophenes†
Abstract
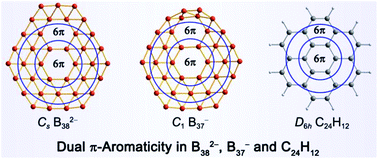
Boron clusters have been found to exhibit a variety of interesting electronic, structural, and bonding properties. Of particular interest are the recent discoveries of the 2D hexagonal B36−/0 which led to the concept of borophenes and the 3D fullerene-like B40−/0 which marked the onset of borospherene chemistry. Here, we present a joint photoelectron spectroscopic and first-principles study of B37− and B38−, which are in the transition size range between the 2D borophene-type clusters and the 3D borospherenes. These two clusters are found to possess highly stable 2D global-minimum structures consisting of a double-hexagonal vacancy. Detailed bonding analyses reveal that both B37− and B38− are all-boron analogues of coronene (C24H12) with a unique delocalized π system, featuring dual π aromaticity. These clusters with double hexagonal vacancies can be viewed as molecular motifs for the χ3-borophene which is the most stable form of borophenes recently synthesized on an Ag(111) substrate.


 Please wait while we load your content...
Please wait while we load your content...