Cu-CDots nanocorals as electrocatalyst for highly efficient CO2 reduction to formate†
Abstract
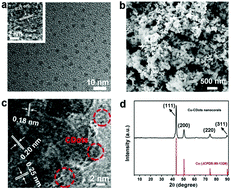
Electrochemical reduction of CO2 is a key component of many prospective artificial technologies for renewable carbon-containing fuels, but it still suffers from the high overpotentials required to drive the process, low selectivity for diversiform products and the high cost of the catalyst. Here, we report that Cu-CDots nanocorals is a highly efficient, low-cost and stable electrocatalyst for CO2 reduction in aqueous solution. The major product of CO2 reduction on the Cu-CDots nanocorals is HCOOH with an inconceivable low overpotential of 0.13 V and a high Faraday efficiency of 79% at a moderate potential of −0.7 V vs. RHE. In the present system, CDots can increase the adsorption capacity of CO2 molecules and H+, which play important roles in CO2 reduction. The high selectivity of HCOOH for CO2 reduction may be ascribed to that CDots can greatly diminish the HCOOH desorption energy and improve the catalytic selectivity for HCOOH. Furthermore, Cu-CDots nanocorals exhibit a long-term stability during 5 h-electrolysis.

 Please wait while we load your content...
Please wait while we load your content...