Cooperation and competition between halogen bonding and van der Waals forces in supramolecular engineering at the aliphatic hydrocarbon/graphite interface: position and number of bromine group effects†
Abstract
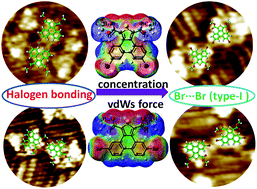
Herein, the photophysical properties of two π-conjugated thienophenanthrene derivatives (6,9- and 5,10-DBTD) are reported. Their self-assembled monolayers in aliphatic hydrocarbon solvents under different concentrations were investigated by scanning tunneling microscopy on a graphite surface. The STM results revealed that the self-assembled structures of the two geometrical isomers exhibited absolutely different behaviors. At the aliphatic solvent/graphite interface, 6,9-DBTD produced almost a single stable coassembled linear structure, except for that with n-tridecane as the solvent. However, the self-assembly of 5,10-DBTD showed structural diversity, and it presented a gradient variety through increasing the chain length of the aliphatic solvents as well as the solution concentration. All ordered self-assembled adlayers critically depend on not only the interchain van der Waals (vdW) interactions, but also on multiple intermolecular interactions, including Br⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and Br⋯S hetero-halogen bonds, homo-Br⋯Br interactions, and H⋯Br and H⋯O hydrogen bonds. We proposed that the cooperation and competition of the intermolecular interactions involving a Br atom and interchain vdW forces induce this structural variety. Density functional theory calculations support to unravel the different elementary structural units based on halogen bonds and hydrogen bonds and were useful tools to dissect and explain the formation mechanism.
C and Br⋯S hetero-halogen bonds, homo-Br⋯Br interactions, and H⋯Br and H⋯O hydrogen bonds. We proposed that the cooperation and competition of the intermolecular interactions involving a Br atom and interchain vdW forces induce this structural variety. Density functional theory calculations support to unravel the different elementary structural units based on halogen bonds and hydrogen bonds and were useful tools to dissect and explain the formation mechanism.


 Please wait while we load your content...
Please wait while we load your content...