Functional nanocomposites with perfect nanoblending between water-soluble polymers and hydrophobic inorganic nanoparticles: applications to electric-stimuli-responsive films†
Abstract
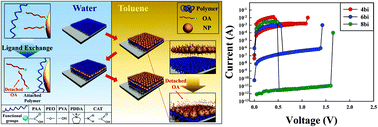
There is rising demand for metal or metal oxide nanoparticle (NP)/polymer nanocomposite films with desired functionalities. However, it is difficult to directly combine well-defined NPs synthesized using organic fatty acids in nonpolar media with water-soluble polymers, except polyelectrolytes with specific functional moieties, because they differ in their hydrophobic/hydrophilic properties. We have developed a facile and universal hydrophilic/hydrophobic layer-by-layer (LbL) assembly method that enables perfect nanoblending between water-soluble polymers and hydrophobic NPs, maintaining their specific functionalities. Various hydrophobic NPs stabilized by using oleic acid (OA) (e.g., OA-TiO2, OA-Fe3O4, OA-Ag, and OA-Pt NPs) were directly LbL assembled with various water-soluble polymers (including biomaterials) containing carboxylic acid (–COOH), tertiary ammonium (N+), hydroxyl (–OH), and/or ether (–O–) groups. This adsorption behavior is based on the affinities between water-soluble polymers with multidentate binding sites and the surfaces of metal or metal oxide NPs stabilized by OA ligands. Our approach can be used to fabricate unipolar switching nonvolatile memory devices with ON/OFF current ratios greater than ∼1010 and good memory stability, despite the use of water-soluble polymers.

 Please wait while we load your content...
Please wait while we load your content...