Promoter effect of hydration on the nucleation of nanoparticles: direct observation for gold and copper on rutile TiO2 (110)†
Abstract
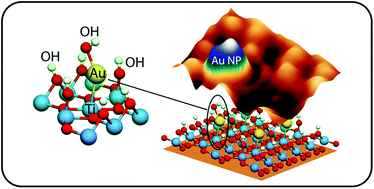
Direct observation of the promoting effect of hydration on the nucleation of gold and copper nanoparticles supported on partially reduced rutile TiO2 (110) is achieved by combined scanning tunneling microscopy experiments and density functional theory calculations. The experiments show a clear difference between the two metals. Gold nanoparticles grow at the vicinity of the surface hydroxyl domains, whereas the nucleation of copper is not substantially affected by hydration. The nucleation of gold on surface oxygen vacancies is observed although this is not the only preferential site. Theoretical calculations of the coadsorbed phases of gold, copper and hydroxyl species on stoichiometric and reduced TiO2 (110) surfaces under relevant conditions of temperature and pressure support the experimental interpretation. Surface hydration tends to stabilize significantly gold adsorption on the stoichiometric support, while its influence on copper adsorption is not pronounced. The theoretical analysis shows that the early stages of the nucleation on hydrated stoichiometric surfaces correspond to mono-hydroxylated metallic species co-chemisorbed with hydroxyl species, whereas those on hydrated reduced surfaces are metallic atoms bound to oxygen vacancies and weakly perturbed by surface hydration.


 Please wait while we load your content...
Please wait while we load your content...