Diameter-selective non-covalent functionalization of carbon nanotubes with porphyrin monomers†
Abstract
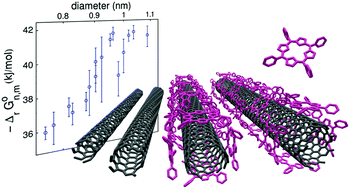
We report on the spontaneous non-covalent functionalization of carbon nanotubes with hydrophobic porphyrin molecules in micellar aqueous solution. By monitoring the species concentrations with optical spectroscopies, we can follow the kinetics of the reaction and study its thermodynamical equilibrium as a function of the reagent concentrations. We show that the reaction is well accounted for by a cooperative Hill equation, reaching a molecular coverage close to a compact monolayer for a porphyrin concentration larger than a diameter-specific threshold concentration. The equilibrium constant is measured for 16 nanotube chiral species. The Gibbs energy of the reaction (of the order of −40 kJ mol−1) and its evolution with the nanotube diameter is consistent with theoretical calculations of the binding energy. This thermodynamical study shows a strong preferential binding of TPP molecules to larger diameter nanotubes. This original curvature selectivity can be used to induce diameter selective species enrichment.


 Please wait while we load your content...
Please wait while we load your content...