Liquid crystal self-assembly of halloysite nanotubes in ionic liquids: a novel soft nanocomposite ionogel electrolyte with high anisotropic ionic conductivity and thermal stability†
Abstract
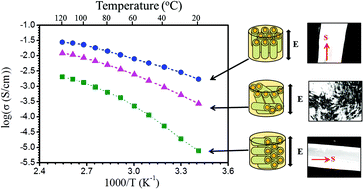
We report a novel class of liquid crystalline (LC) nanohybrid ionogels fabricated via self-assembly of natural halloysite nanotubes (HNTs) in ionic liquids (ILs). The obtained ionogels are very stable and nonvolatile and show LC phases over a wide temperature range. Remarkably, the nanocomposite ionogels exhibit high anisotropic ionic conductivity after shear, and their room temperature ionic conductivity can reach 3.8 × 10−3 S cm−1 for aligned nanotubes perpendicular to the electrode even when the HNTs content increases to 40 wt%, which is 380 times higher than that obtained for aligned nanotubes parallel to the electrode, which is 1.0 × 10−5 S cm−1. Crucially, the obtained LC nanocomposite ionogels have very high thermal stability, which can sustain 400 °C thermal treatment. The findings will promote the development of novel nanocomposite ionogel electrolytes with faster ion transport and larger anisotropic conductivity.

 Please wait while we load your content...
Please wait while we load your content...