Transmembrane delivery of anticancer drugs through self-assembly of cyclic peptide nanotubes†
Abstract
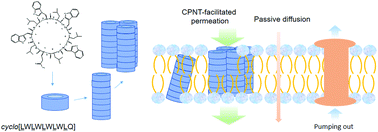
Breaking the natural barriers of cell membranes achieves fast entry of therapeutics, which leads to enhanced efficacy and helps overcome multiple drug resistance. Herein, transmembrane delivery of a series of small molecule anticancer drugs was achieved by the construction of artificial transmembrane nanochannels formed by self-assembly of cyclic peptide (cyclo[Gln-(D-Leu-Trp)4-D-Leu], CP) nanotubes (CPNTs) in the lipid bilayers. Our in vitro study in liposomes indicated that the transport of molecules with sizes smaller than 1.0 nm, which is the internal diameter of the CPNTs, could be significantly enhanced by CPNTs in a size-selective and dose-dependent manner. Facilitated uptake of 5-fluorouracil (5-FU) was also confirmed in the BEL7402 cell line. On the contrary, CPs could facilitate neither the transport across liposomal membranes nor the uptake by cell lines of cytarabine, a counterevidence drug with a size of 1.1 nm. CPs had a very weak anticancer efficacy, but could significantly reduce the IC50 of 5-FU in BEL7402, HeLa and S180 cell lines. Analysis by a q test revealed that a combination of 5-FU and CP had a synergistic effect in BEL7402 at all CP levels, in S180 at CP levels higher than 64 μg mL−1, but not in HeLa, where an additive effect was observed. Temporarily, intratumoral injection is believed to be the best way for CP administration. In vivo imaging using 125I radio-labelled CP confirmed that CPNPTs were completely localized in the tumor tissues, and translocation to other tissues was negligible. In vivo anticancer efficacy was studied in the grafted S180 solid tumor model in mice, and the results indicated that tumor growth was greatly inhibited by the combinatory use of 5-FU and CP, and a synergistic effect was observed at CP doses of 0.25 mg per kg bw. It is concluded that facilitated transmembrane delivery of anticancer drugs with sizes smaller than 1.0 nm was achieved, and the synergistic anticancer effect was confirmed both in cell lines and in vivo through the combinatory use of 5-FU and CP.

 Please wait while we load your content...
Please wait while we load your content...