Theranostic reduction-sensitive gemcitabine prodrug micelles for near-infrared imaging and pancreatic cancer therapy†
Abstract
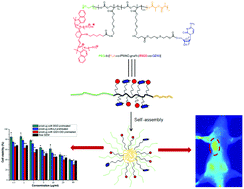
A biodegradable and reduction-cleavable gemcitabine (GEM) polymeric prodrug with in vivo near-infrared (NIR) imaging ability was reported. This theranostic GEM prodrug PEG-b-[PLA-co-PMAC-graft-(IR820-co-GEM)] was synthesized by ring-opening polymerization and “click” reaction. The as-prepared reduction-sensitive prodrug could self-assemble into prodrug micelles in aqueous solution confirmed by dynamic light scattering (DLS) and transmission electron microscopy (TEM). In vitro drug release studies showed that these prodrug micelles were able to release GEM in an intracellular-mimicking reductive environment. These prodrug micelles could be effectively internalized by BxPC-3 pancreatic cancer cells, which were observed by confocal laser scanning microscopy (CLSM). Meanwhile, a methyl thiazolyl tetrazolium (MTT) assay demonstrated that this prodrug exhibited high cytotoxicity against BxPC-3 cells. The in vivo whole-animal near-infrared (NIR) imaging results showed that these prodrug micelles could be effectively accumulated in tumor tissue and had a longer blood circulation time than IR820-COOH. The endogenous reduction-sensitive gemcitabine prodrug micelles with the in vivo NIR imaging ability might have great potential in image-guided pancreatic cancer therapy.

 Please wait while we load your content...
Please wait while we load your content...