TiO2/vanadate (Sr10V6O25, Ni3V2O8, Zn2V2O7) heterostructured photocatalysts with enhanced photocatalytic activity for photoreduction of CO2 into CH4†
Abstract
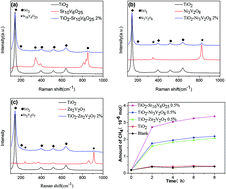
A series of TiO2/vanadate (Sr10V6O25, Ni3V2O8, Zn2V2O7) heterostructured photocatalysts were prepared by a simple sol–gel method. The theoretical calculations imply the possible energy band match between TiO2 and vanadates. Characterized by XRD, Raman, TEM, EDX, XPS, absorption spectra, PL and time-resolved PL decay curves, it is revealed that the vanadates, which exist on the surface of TiO2, could suppress the recombination of charge carriers, prolong the life-time of photogenerated electrons and provide surface reactive hole sites, improving the photocatalytic activity for photo-reduction of CO2 into CH4.

 Please wait while we load your content...
Please wait while we load your content...