Entropy–enthalpy compensation at the single protein level: pH sensing in the bacterial channel OmpF†
Abstract
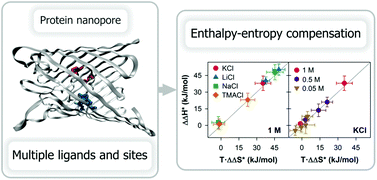
The pH sensing mechanism of the OmpF channel operates via ligand modification: increasing acidity induces the replacement of cations with protons in critical binding sites decreasing the channel conductance. Aside from the change in enthalpy associated with the binding, there is also a change in the microscopic arrangements of ligands, receptors and the surrounding solvent. We show that the pH-modulation of the single channel conduction involves small free energy changes because large enthalpic and entropic contributions change in opposite ways, demonstrating an approximate enthalpy–entropy compensation for different salts and concentrations.


 Please wait while we load your content...
Please wait while we load your content...