Highly photoluminescent silicon nanocrystals for rapid, label-free and recyclable detection of mercuric ions†
Abstract
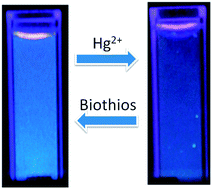
Hydrothermal treatment of 3-aminopropyltrimethoxysilane (APTMS) in the presence of sodium citrate generates a suspension of highly fluorescent silicon nanocrystals that fluoresces blue under UV irradiation. The photoluminescent quantum yield of the as-prepared silicon nanocrystals was calculated to be 21.6%, with quinine sulfate as the standard reference. Only mercuric ions (Hg2+) can readily prevent the fluorescence of the silicon nanocrystals, indicating a remarkably high selectivity towards Hg2+ over other metal ions. The optimized sensor system shows a sensitive detection range from 50 nM to 1 μM and a detection limit of 50 nM. The quenching mechanism was explained in terms of optical absorption spectra and time-resolved fluorescence decay spectra. Due to the strong interaction of Hg2+ with the thiol group, the fluorescence can be fully recovered by biothiols such as cysteine and glutathione, therefore, a regenerative strategy has been proposed and successfully applied to detect Hg2+ by the same sensor for at least five cycles. Endowed with relatively high sensitivity and selectivity, the present sensor holds the potential to be applied for mercuric assay in water.

 Please wait while we load your content...
Please wait while we load your content...