From isotope labeled CH3CN to N2 inside single-walled carbon nanotubes
Abstract
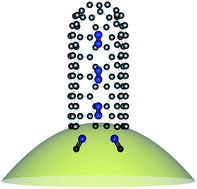
The observation of one-dimensional N2 inside single-walled carbon nanotubes raises the questions, how are the N2 molecules formed and how do they manage to make their way to this peculiar place? We have used N15 and C13 isotope labeled acetonitrile during the synthesis of single-walled carbon nanotubes to investigate this process. The isotope shifts of phonons and vibrons are observed by Raman spectroscopy and X-ray absorption. We identify the catalytic decomposition of acetonitrile as the initial step in the reaction pathway to single-walled carbon nanotubes containing encapsulated N2.

 Please wait while we load your content...
Please wait while we load your content...