Surface functional groups of carbon nanotubes to manipulate capacitive behaviors†
Abstract
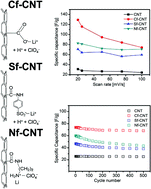
The covalent functionalization of carbon nanotubes (CNTs) is a basic but important chemistry that can modify their physicochemical properties, resolve their poor dispersion capability, and improve their capacitance to enable their use as high-energy supercapacitors. However, the relationship between functional groups on the CNT surface and their capacitive characteristics has not yet been explored. Here, we demonstrate the influence of carboxylic, sulfonic, and amine groups tethered to CNTs (Cf-CNTs, Sf-CNTs, and Nf-CNTs, respectively) on capacitor performance in an organic electrolyte. The Cf-CNTs show the highest specific capacitance of 129.4 F g−1, four-fold greater than 31.2 F g−1 of pristine CNTs, but they reveal the lowest rate capability of 57%. In contrast, the Sf- and Nf-CNTs exhibit specific capacitances of 70.9 F g−1 and 83.6 F g−1, two-fold greater than that of pristine CNTs, along with a good rate capability greater than 80%. Despite their pseudocapacitive nature, all functionalized CNTs show a cyclic stability of more than 80% after 500 cycles due to the electrochemical stability of the functional groups. As demonstrated by spectroscopic analysis, the supercapacitive behaviors of the functionalized CNTs originate from specific interactions between functional groups and lithium ions and the alteration of the electronic structure arising from covalent functionalization.

 Please wait while we load your content...
Please wait while we load your content...