Mesoporous (ZnO)x(MgO)1−x nanoplates: template-free solvothermal synthesis, optical properties, and their applications in water treatment†
Abstract
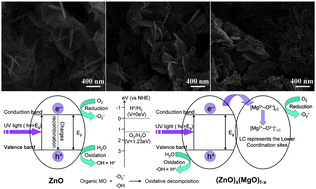
Mesoporous (ZnO)x(MgO)1−x nanoplates were synthesized from a solution containing zinc acetate and magnesium acetate by a template-free solvothermal synthetic method followed by subsequent calcination. After thermal treatment, the plate-like morphology was retained. The formation of pores was due to thermal decomposition of Mg(OH)2 and the release of H2O. The optical properties of the mesoporous (ZnO)x(MgO)1−x nanoplates had been investigated by UV-vis absorption and cathodoluminescence (CL) emission spectroscopy. The UV-vis absorption spectra showed the band gap variation of the as-prepared samples due to the presence of ZnO in the MgO nanostructures. The CL spectra showed strong broad peaks in the visible range from 450 to 700 nm, indicating significant oxygen vacancy defects on the surface of the (ZnO)x(MgO)1−x nanoplates. Moreover, the samples were evaluated as photocatalysts for the UV-induced degradation of methyl orange (MO) in aqueous solution. The (ZnO)x(MgO)1−x nanoplates showed high photocatalytic performance and thus would be promising candidates for polluted water treatment.

 Please wait while we load your content...
Please wait while we load your content...