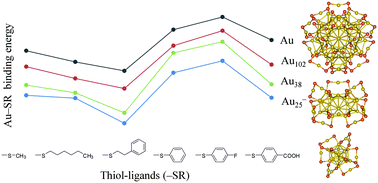
We have studied the electrochemical and thermodynamic stability of Au25(SR)18−, Au38(SR)24, and Au102(SR)44, R = CH3, C6H13, CH2CH2Ph, Ph, PhF, and PhCOOH, in order to examine ligand effects on the stability of thiol-stabilized gold nanoclusters, Aum(SR)n. Aliphatic thiols, in general, have higher electrochemical and thermodynamic stability than aromatic thiols, and the –SCH2CH2Ph thiol is particularly appealing because of its high electrochemical and thermodynamic stability. The stabilization of Aum by nSR for Aum(SR)n can be rationalized by the stabilization of an Au atom by an SR for the simple molecule AuSR, regardless of interligand interaction and system size and shape. Thiol moieties play a strong role in the electron oxidation and reduction of Aum(SR)n. Accounting for the characteristics of thiol ligands is essential for understanding the electronic and thermodynamic stability of thiol-stabilized gold nanoclusters.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...